e-Myco™ VALiD Mycoplasma PCR Detection Kit

e-Myco™ VALiD Mycoplasma PCR Detection Kit is a product for the verification of mycoplasma contamination in biological materials.
This kit is designed to detect the presence of mycoplasma that might contaminate biological materials such as cultured cells. Also, the kit can be performed in 3 hours, can detect sensitively until 10 CFU/ml and include internal control for verifying a PCR run as well as positive control DNA.
• Ready to use
• Time Save
• Smart (internal control and 8-MOP)
• Steady (Broad Species Detection)
• Stage-up (Sensitive and reliable)• Mycoplasma contamination monitoring in cell-based pharmaceutical production (pre-, during, and post-process)
• Contamination control of cell lines
| No. | Kit Contents | Unit |
|---|---|---|
| 1 | e-Myco™ VALiD Mycoplasma PCR Premix
< 0.01% Hot start Taq DNA Polymerase < 0.01% dATP, dTTP, dGTP, dCTP < 0.005% Mycoplasma Primers, Internal Control < 0.001% 8-MOP (dissolved in DMSO) |
48T |
| 2 | Positive Control
< 0.01% recombinant DNA included partial 16S sequence of M. hyorhinis |
25 μl x 3Tubes |
| 3 | DNase/RNase Free Water
No template control < DNase/RNase Free Water |
1 ml x 1Tube |
• Should be stored below -20ºC after receiving
• e-Myco™ VALiD Mycoplasma PCR Detection Kit can be stored for up to 18 months without showing any reduction in performance and quality under appropriate storage condition
• The expiration date is labeled on the product box
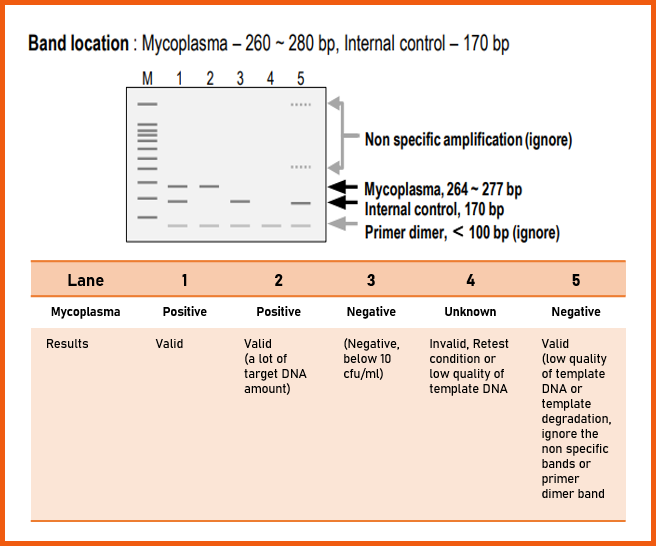
1. Interpretation
e-Myco™ VALiD Mycoplasma PCR Detection Kit provides the detection of various Mycoplasma
species with high sensitivity and specificity.
For the validation of each PCR reaction, it includes internal control in each PCR Premix tube. The interpretation of experimental results is as follows.
2. Specificity Test
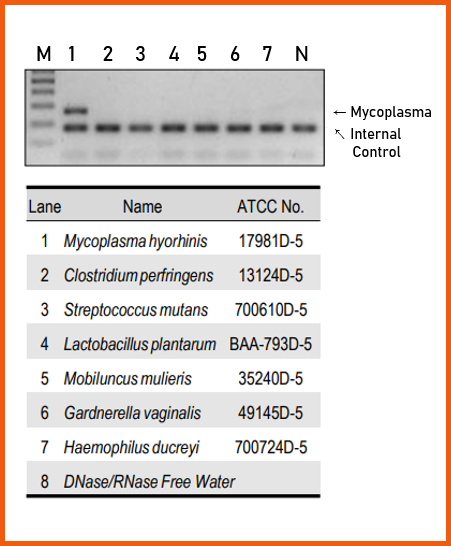
To verify the detection specificity of this product for Mycoplasma, amplification was performed using DNA from six non-Mycoplasma bacterial species and a positive control, M. hyorhinis.
In M. hyorhinis, both the target band and the internal control band were amplified, whereas in the six bacterial DNA samples and the no-template control, only the internal control band was detected.
These results confirm that the e-Myco™ VALiD Mycoplasma PCR Detection Kit specifically detects Mycoplasma.
Fig.2 shows the evaluation data of e-Myco™ VALiD Mycoplasma PCR Detection Kit, suggesting that both internal control (app. 180 bp) and target bands (app. 270 bp) were detected.
However, in cases of negative control(20 ng of non-mycoplasma bacterial genomic DNA, lanes 2~7) and no template control (lane N), only negative control band was detected.
3. Analytical Sensitivity
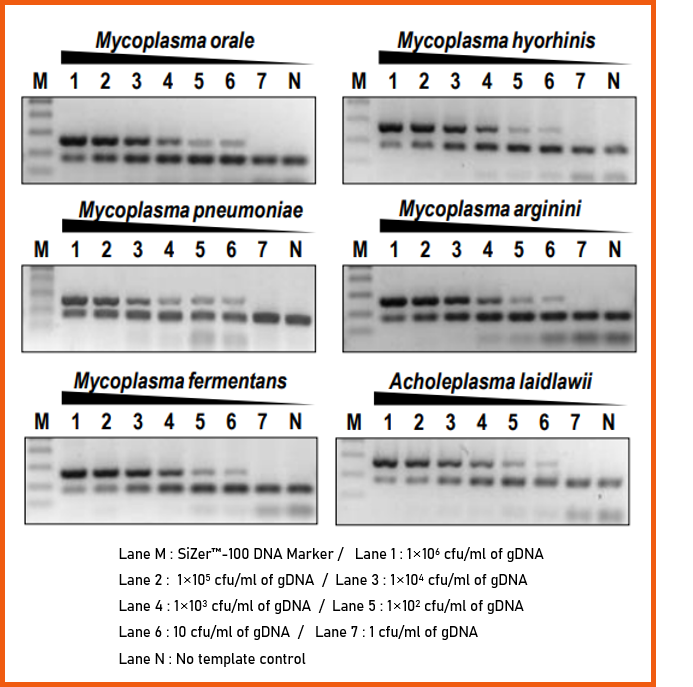
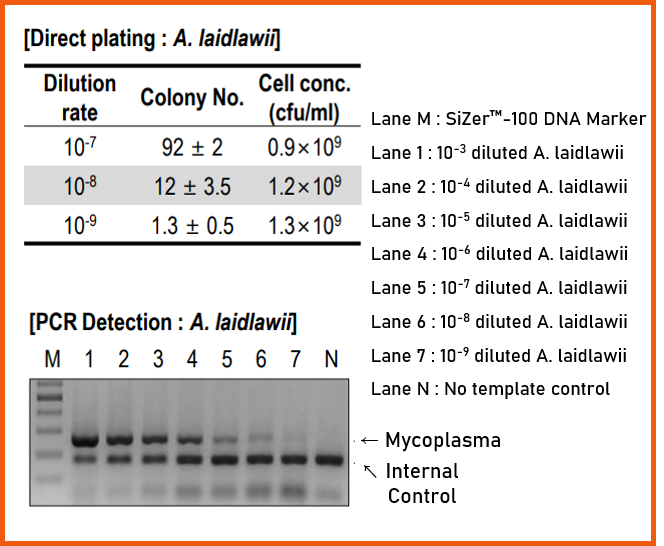
e-Myco™ VALiD Mycoplasma PCR Detection Kit is an eligible kit for the efficient detection of Mycoplasma spp. contamination with high sensitivity in the culture.
To identify the analytical sensitivity, the genomic DNA from cell cultured 6 Mycoplasma spp. were purified. The sensitivity according to the DNA copy number was investigated after purifying gDNA from each cultured Mycoplasma spp.
4. Comparison with Direct Plating Method
| Product | Cat.No | Capacity | inquire | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiZer™-100 DNA Marker Solution Best |
|
|||||||||
| SiZer™-1,000 plus DNA Marker Solution |
|
|||||||||
| G-spin™ Total DNA Extraction Mini Kit Best |
|
|||||||||