LiliF® BVDV RT-PCR Kit
Bovine rotaviruses cause diarrhea during infections due to diseases that are problematic in the newborn period in a variety of animals, including cows. In Korea, it is one of the most harmful digestive diseases in young newborn calves. It is infected mainly by young cows less than 1 week old and causes persistent diarrhea, resulting in high mortality when lasting more than 3 days. The causative agent is Reoviridae rotavirus, a double-stranded RNA virus consisting of 11 segments, and the name rotavirus was attached to the two-layer capsid when viewed under an electron microscope. According to the antigenicity, 7 groups are classified into A to G, and 3 groups of A to C are reported as cow rotavirus. Radio waves are made up of oral feces in the feces, that is, the feces containing the virus are made directly or through the oral contaminants that are contaminated with the virus. In addition, there is a possibility that the infectious virus of the moth may be released into the feces and act as an infectious agent. During the administration of the cattle, the feces of the infected cattle are buried in boots and mechanically propagated to other cattle's cows or even propagated to other farms Sometimes. Symptoms include severe milky white diarrhea and sudden dehydration symptoms in infants younger than 1 week of age and most of them do not improve antibiotic treatment. In case of calves over 1 month old, symptoms are not severe In some cases, the symptom therapy can be improved easily. The lesion is confined to the small intestine. Histopathologically, the enlargement of the chorionic villi epithelium, degeneration and detachment are seen. Generally, in the case of large farms, the seasonal delivery program is performed or the interval of delivery is narrow. The pattern is more explosive than in small farms, especially from late winter to early spring. It is impossible to diagnose by clinical symptoms alone and various diseases and differential diagnosis are needed. Since feces in the early stages of onset contain a large amount of virus, it is necessary to detect viral or gene directly in feces or try to isolate virus using cultured cells However, since it takes a very long time to isolate the virus through cultured cells, molecular diagnosis is required for rapid diagnosis.
LiliF™ BVDV RT-PCR Kit is able to detect directly and specifically Bovine Viral Diarrhea virus by CLP™ technology and Maxime ™ technology on the basis of a genetic database of target nucleic acid fragments. Therefore, this kit can diagnose very sensitive, fast and accurately.
The kit contains a specific primer set for a highly conserved region based on current sequence alignments of Bovine Viral Diarrhea virus, allowing the RNA detection. It can determine the infecting all serotype and accurately and sensitivity detect multiple detection genes at one time using the conventional RT-PCR method, and take only 2 hours for detection. Fast and sensitive detection of pathogen enables patients to get appropriate treatment and prevent the rapid spreading of disease by separating patients immediately.
• This product is a qualitative RT-PCR testing product with CLP™ technology which provided flexibility in Tm (melting temperature) of primer design for optimization of reaction condition, and maximizes PCR specificity and sensitivity through the control of non-specific priming.
• The assay is a real-time RT-PCR that discriminates Bovine Viral Diarrhea virus in one reaction. The assay is composed of two principal steps: (1) nucleic acid extraction from specimens, and (2) amplification of the target extracted nucleic acid fragment using specific primers pair.
• The assay can be detect Bovine viral diarrhea virus type-1 and type-2, Border disease virus and Classical swine fever virus by one-step RT-PCR.
• IVD Reagents for molecular genetics for non-legally designed infectious pathogen by OIE
• Permission No. 133-016 of Medical Devices for veterinary use authorized by Animal and Plant Quarantine Agency of Korea
• This kit is developed, designed, and sold for IVD purpose.
• This product is research reagent of infectious disease for professional use to restrict the public use for animal diseases.
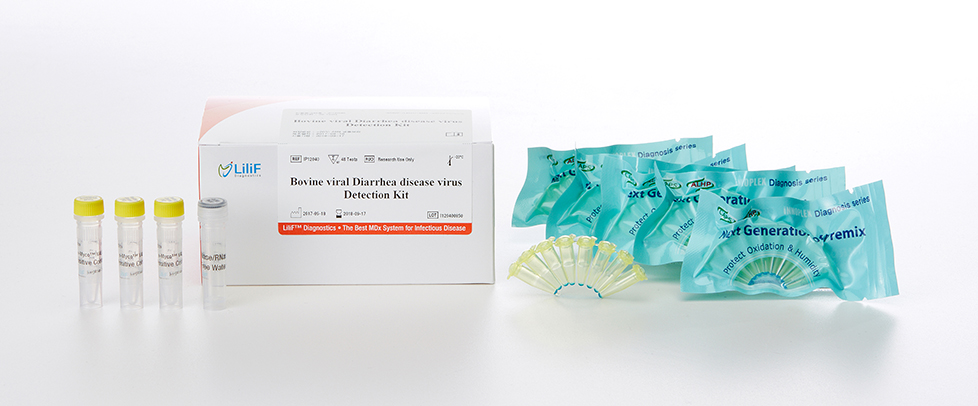
| No. | Contents | 48 Tests/Kit |
|---|---|---|
| 1 | BVDV Detection Premix | 48 tubes |
| 2 | BVDV Positive Control | 25 μl x 3 tubes |
| 3 | DNase/RNase Free Water | 1 ml x 1 tube |
| Product | Cat.No | Capacity | inquire |
|---|