LiliF® COVID-19 Multi Real-time RT-PCR Kit
There are four genes in the Coronavirus family. Those are known to alpha, beta, gamma, and delta. Alpha and beta corona viruses can cause illness in both humans and animals, whereas others, such as gamma and delta coronaviruses, only infect animals.
Accordingly, in order to increase the speed, accuracy, and convenience of molecular diagnosis for the new coronavirus, a product capable of simultaneously detecting RdRP, N and E genes specific to the new coronavirus was designed.
The new coronavirus (COVID-19) belongs to beta and is one of the new infectious corona viruses that infects the human body as a pathogen of mass pneumonia that occurred in Wuhan, Hubei, China in December 2019. It is very important to diagnose an infection quickly, because there are no vaccines or antivirals approved for prophylactic or therapeutic purposes.
Accordingly, in order to increase the speed, accuracy, and convenience of molecular diagnosis for the new coronavirus, a product capable of simultaneously detecting RdRP, N and E genes specific to the new coronavirus was designed.
• LiliF™ COVID-19 Multi Real-time RT-PCR Kit can detect the new coronavirus using probe method of Real-time RT-PCR, through the reacting of the specific primer and Fluorescent probe in sample. LiliF™ COVID-19 Multi Real-time RT-PCR Kit can detect RdRP and E gene, markers for detecting new coronaviruses. Also, N gene suggested by the US CDC and RNaseP gene which can confirm the validity of all test reactions are adopted and designed for simultaneous detection.
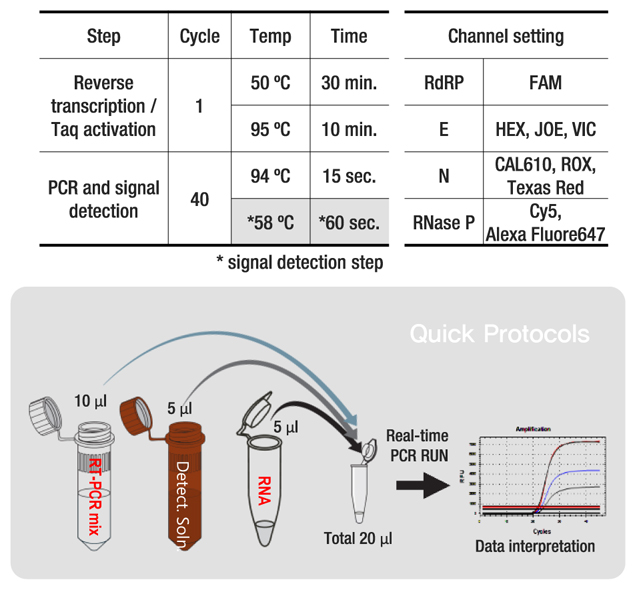
• LiliF™ COVID-19 Multi Real-time RT-PCR Kit is in vitro diagnostic medical device based on real-time reverse transcription PCR method intended for the qualitative detection of nucleic acid from the 2019-nCoV in nasopharyngeal/oropharyngeal swabs and sputa from individuals with signs and symptoms of infection who are suspected of COVID-19
| No. | Contents | 50 tests/kit |
|---|---|---|
| 1 | 2X RT-PCR Mix | 1100 μl x 1 tube |
| 2 | COVID Multi Detection Solution | 550 μl x 1 tube |
| 3 | Positive Control | 150 μl x 1 tube |
| 4 | DNase/RNase Free Water(Negative Control) | 1mll x 1 tube |
| 1 | Vacunas | Discordant results of SARS-CoV-2 PCR-based tests in the early phase of pandemic in Indonesia: Infection control consequences | A.Pranata, B.Zulkifli, S.F.Santosa, A.Oktiviyari | Indonesia |
| 2 | MOH, Thailand, Vol. 62 No. 3 (2020): July - September 2020 | Pre-marketing Evaluation of real-time RT PCR Test Kits for SARS-CoV-2 Detection | Don Changsom, Siriphan Saeng-aroon, Thanutsapa Thanadachakul | Thailand |
| 3 | Salud, Barranquilla vol.36 no.1 Barranquilla Jan./Apr. 2020 Epub May 25, 2021 | SARS-CoV-2 (COVID-19): state of the pandemic, clinical scenarios, strategies for the health sector and its bioethical aspects | SERGIO VERGARA CÁRDENAS, ANDREINA ZANNIN FERRERO, LUIS GUSTAVO CELIS REGALADO | Columbia |
| Product | Cat.No | Capacity | inquire | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patho Gene-spin™ DNA/RNA Extraction Kit Best |
|
|||||||||
| Miracle-AutoXT Automated Nucleic Acid Extraction System Best |
|
|||||||||