LiliF® ASFV Real-time PCR Kit
Animal Medical Device No. 133-25 African Swine Fever Virus Diagnostic Kit
• Veterinary Medical Device No. 133-25 Reagent for high-risk animal infectious disease genetic testing
• Improved detection performance through ASFV standard diagnostic method and optimization of our company's 2x PCRmix
• Detection performance for URL-ASF reference DNA and NCBI published ASFV sequences
• Realization of high specificity for product-like diseases of verification
• Excellent clinical sensitivity and specificity in diagnostic clinical samples (whole blood, serum, tissue)
• Mastermix type product with high versatility for PCR equipment compared to existing products and long-term (1 year) storage.
LiliF® ASFV Real-time PCR Kit was approved as a veterinary medical device by the Quarantine Headquarters (Permission Information: Animal Medical Device No. 133-25 High Risk Animal Contagious Disease
As a product obtained from genetic test reagent), the target gene, p72 gene, can be qualitatively detected by real-time polymerase chain reaction (Real-time PCR).
This is a product that has been implemented.
This product is optimized with our own 2x PCR mix by adopting the primers and probes of the ASFV standard diagnostic method presented by the World Organization for Animal Health (WOAH)
This product realizes fast detection performance through This product was developed based on the p72 gene of ASFV type 2, which mainly occurs in the Asian continent.
This product confirmed the detection limit of 2.5 copy/uL through an analytical performance test.
We validated the product's inclusivity performance using synthetic DNA and URL-ASF reference DNA for genotyping. Perception in ASFV Molecular Diagnosis
African swine fever virus that has no cross-reactivity to 7 types of similar diseases that require a star and no interference reactivity to exogenous or endogenous interfering substances
It is a product with high specificity for Internal positive control (IPC) DNA and IPC that do not affect target detection are included in this product.
By adding specific primers/probes, IPC detection can be confirmed regardless of target amplification, so the validity of the PCR reaction can be confirmed.
• IVD Reagent for molecular genetics for veterinary use
• For Research Use Only, Not for use in diagnostic procedures
• This product is research reagent of infectious disease for professional use to restrict the public use for animal diseases
| No. | Contents | 50 tests/kit |
|---|---|---|
| 1 | 2X PCR Mix | 560 μl x 1 tube |
| 2 | ASFV Detection Solution | 280 μl x 1 tube |
| 3 | Positive Control | 25 μl x 1 tube |
| 4 | DNase/RNase Free Water(Negative Control) | 1mll x 1 tube |
• Analytical detection performance evaluation of ASFV p72 gene (genotype II) of the LiliF® ASFV Real-time kit.
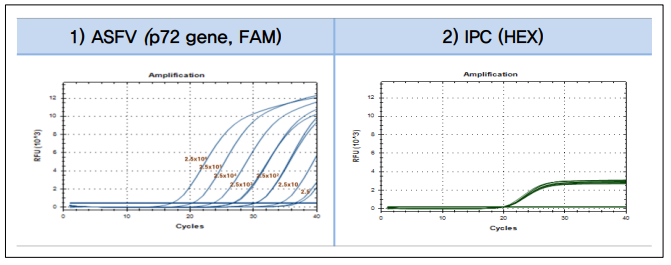
Fig 1. Analytical detection performance evaluation of ASFV p72 gene (genotype II) of the LiliF® ASFV Real-time kit.
1) LiliF® ASFV Real-time Kit is detectable up to 2.5 copy/uL (p72 genotype II). 2) PCR reaction validation would be
checked through IPC DNA amplification and detection.
• Verification of strain reactivity of the LiliF® ASFV Real-time PCR kit for various p72 genotypes.
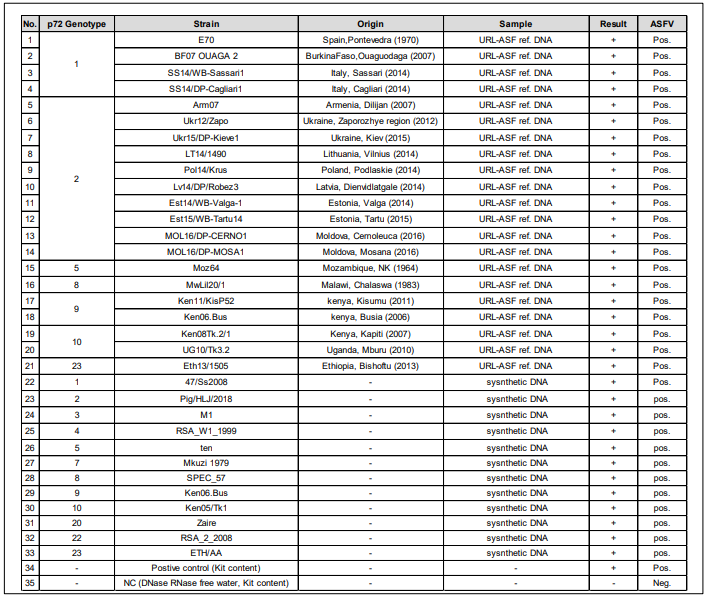
Fig 2. Verification of strain reactivity of the LiliF® ASFV Real-time PCR kit for various p72 genotypes. Using 21 URL-ASF reference DNAs from
ASF-EURL and synthetic DNA of ASFV p72 gene published in NCBI database detection were verified. The kit was verified that ASFV
detection was possible in a total of 33 test samples including various p72 genotypes
• Analytical Specificity Performance Verification for ASFV of the LiliF® ASFV Real-time PCR kit.
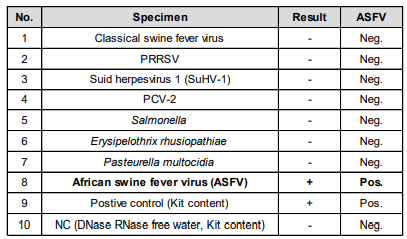
Fig 3. Analytical Specificity Performance Verification for ASFV of the LiliF® ASFV Real-time PCR kit. The specificity of ASFV of the LiliF® ASFV
Real-time PCR Kit was evaluated using nucleic acids from seven swine diseases taken from the Clinical specimens. Negative results were confirmed
in seven types of nucleic acids excluding ASFV DNA (No.8), and as a result, the specificity of the kit was verified.
| Product | Cat.No | Capacity | inquire |
|---|