FaSTAR-XT PGS DNA/RNA Kit (I)

The FaSTAR-XT PGS DNA/RNA Kit (I) is designed for efficient and reliable extraction of viral RNA and DNA from various samples. The FaSTAR-XT PGS DNA/RNA Kit (I) utilizes a streamlined process that minimizes contamination risks and maximizes yield, making it suitable for both clinical testing and research applications.
Each cartridge is pre-loaded with the necessary reagents, simplifying the workflow. Users can easily insert samples into the cartridge, which is designed to be compatible with various sample types, including swabs, whole blood, stool and etc. The FaSTAR-XT PGS DNA/RNA Kit is designed to effectively remove impurities and inhibitors, allowing for the extraction of high-quality nucleic acids. One of the key advantages of this kit is its ability to provide rapid results. The extraction process can be completed in under 12 minutes with a rapid protocol, making it ideal for sensitive applications such as research on viral infections. Additionally, the individual cartridge design facilitates handling and reduces the risk of cross-contamination between samples. This kit is compatible with downstream applications such as PCR and qPCR, ensuring that the extracted nucleic acids can be used for a variety of analyses. Its robust performance has been validated across different viral targets, demonstrating high sensitivity and specificity.
• High/Low throughput : The well plate type allows for high throughput processing, while the individual type can process 1 or more low throughput samples, so you can test 1 to 48 samples simultaneously.

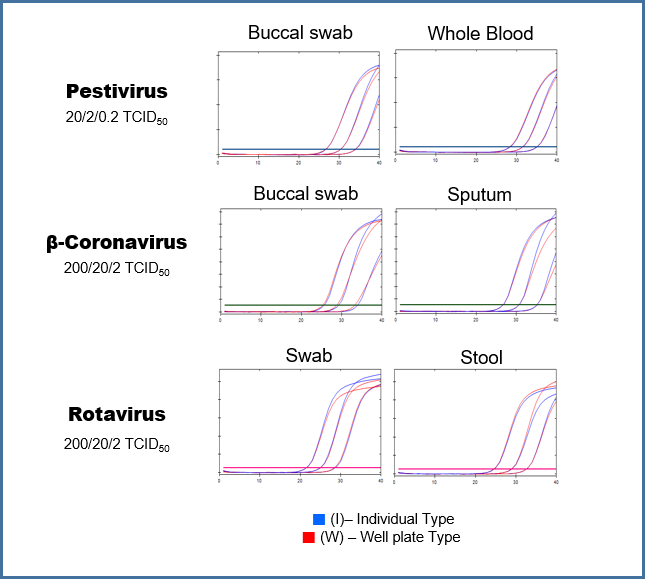
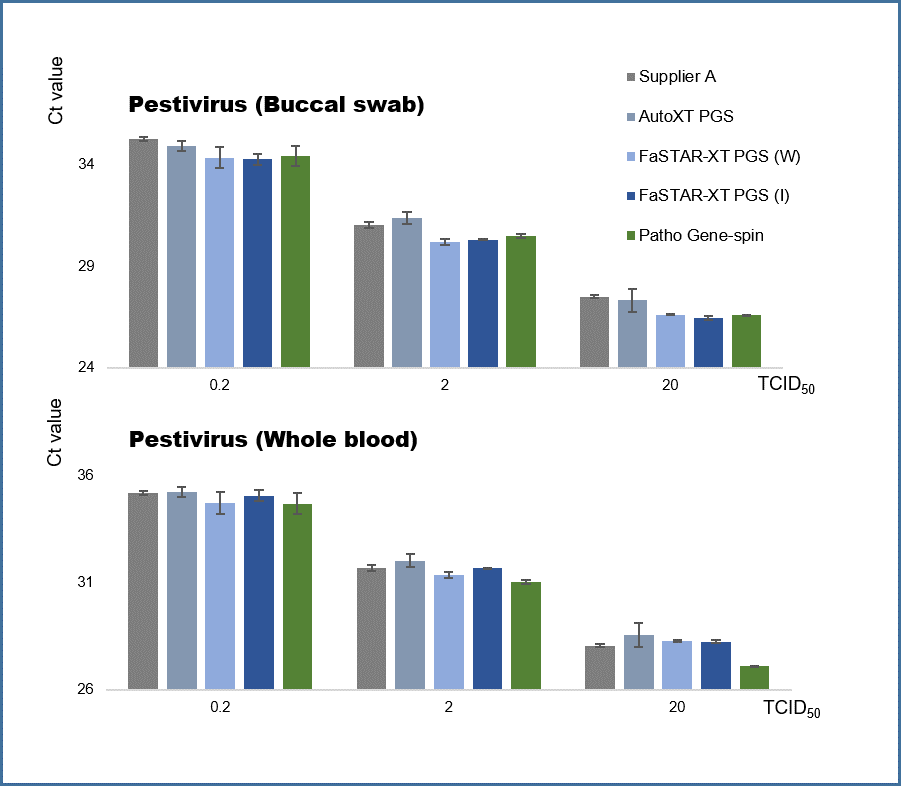
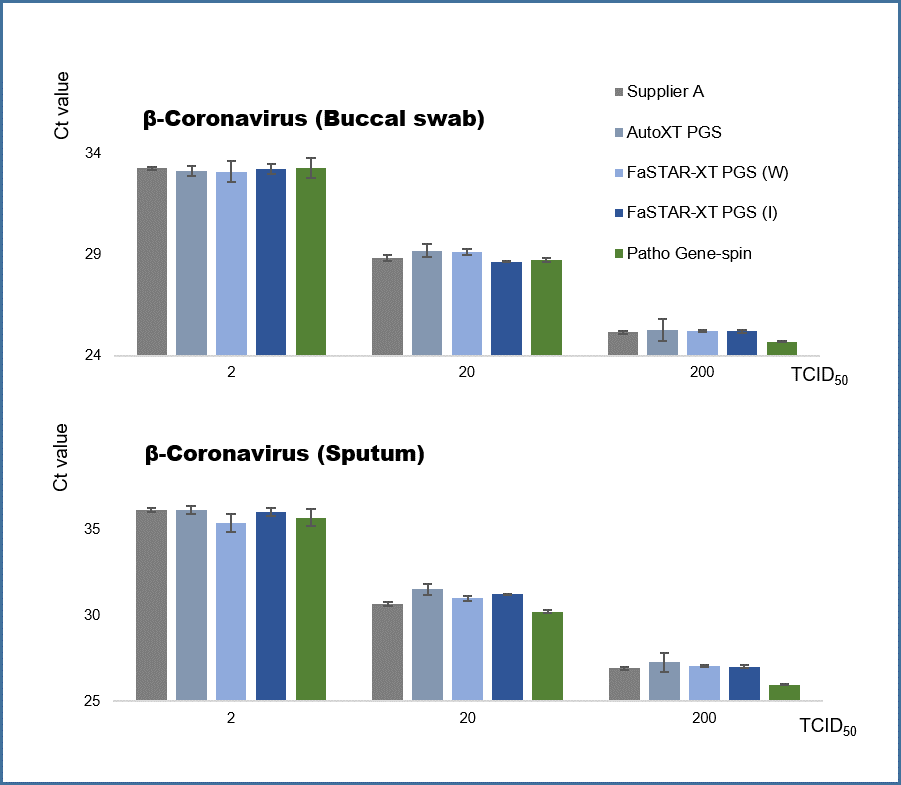
Comparative results of the extraction performance of β-Coronavirus.
The comparative performance results for β-Coronavirus extraction show that the FaSTAR-XT PGS DNA/RNA kit (I) provides stable extraction performance for enveloped viruses. When evaluated using β-coronavirus spike samples (buccal swabs and sputum), the kit demonstrated equivalent extraction performance compared to other products and manual extraction methods, effectively confirming the detection of the virus.
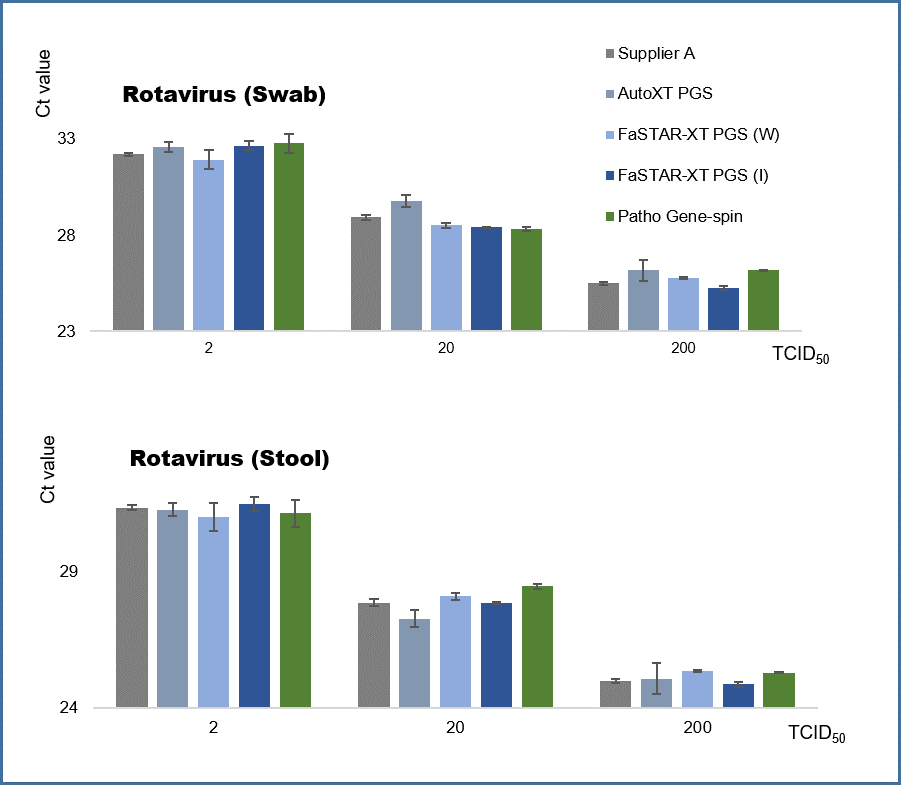
Comparative results of the extraction performance of Rotavirus
The FaSTAR-XT PGS DNA/RNA kit (I) provides stable extraction performance for non-enveloped viruses. The extraction performance was compared for the non-enveloped virus, rotavirus. In the evaluation using rotavirus spike samples (swabs and stool), this kit demonstrated equivalent extraction performance compared to other products and manual extraction methods, effectively confirming the detection of the virus. It delivers accurate test results for both high and low concentration samples.
• The FaSTAR-XT PGS DNA/RNA Kit (I) is designed to effectively prevent cross-contamination between samples. By using an automated extraction system and special disposable tubes, the risk of external contamination is minimized, ensuring reliable results in research and diagnostics.
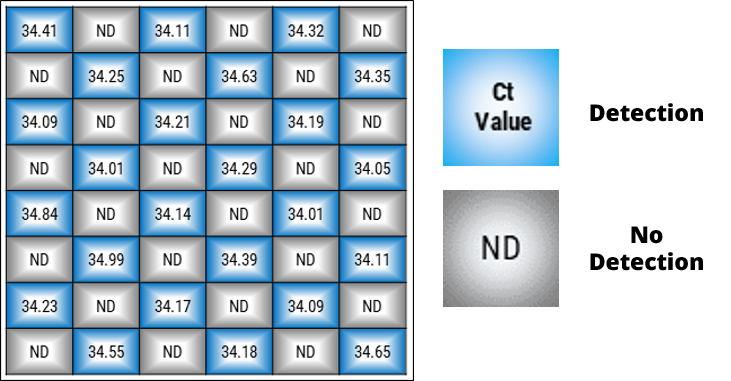
Cross-contamination verification test results
Repeated tests using Pestivirus (0.2 TCID50) samples confirmed consistent positive detection and uniform Ct values in the wells with samples, with no detection in non-input wells.
| Product | Cat.No | Capacity | inquire | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FaSTARprep96 Automated Nucleic Acid Extraction System Best |
|
||||||||||||
| FaSTAR-XT PGS DNA/RNA Kit (W) |
|
||||||||||||
| Patho Gene-spin™ DNA/RNA Extraction Kit Best |
|
||||||||||||
| RealMOD™ Probe R² 2X qPCR mix (with UDG) Best |
|
||||||||||||
| RealMOD™ Probe R² 2X qRT-PCR mix (with UDG) Best |
|
||||||||||||