e-Myco™ VALiD-Q Real-time PCR Kit (ver. 2.0)

e-Myco VALiD-Q Real-time PCR Kit (ver2.0) is designed to specifically detect mycoplasma by targeting highly conserved regions of Mycoplasma DNA.
The e-Myco™ VALiD-Q Real-time PCR Kit (ver. 2.0) is suitable for rapid and accurate testing of mycoplasma negative results, providing confirmation within 1 hour (52 minutes).
The e-Myco™ VALiD-Q Real-time PCR Kit (ver. 2.0) is an in vitro nucleic acid amplification test optimized for the detection of Mycoplasma in K562 cell culture, according to the Nucleic Acid Amplification Tested (NAT) guidelines for Mycoplasma described in chapter 2.6.7 of the European Pharmacopoeia, with respect to specificity, sensitivity/detection limit, and robustness.
The kit was developed according to the CFU specifications provided by suppliers of test materials (e.g., European Directorate for the Quality of Medicines & Health Care and Ministry of Food and Drug Safety).
• Amplification : The extracted DNA is amplified using a 5’nuclease fluorescent probe and specific primer pairs.
• Target Regions : The assay targets two specific regions: Mycoplasma (FAM) and Internal Positive Control (IPC) (HEX).
• Purpose of IPC : The IPC is included in the kit to confirm the success of the Real-time PCR reaction and is co-amplified with the target band from test samples.
• Mycoplasma validation for the production of biopharmaceuticals.
• Rapid contamination detection using real-time PCR : 52 min
• Prevention of carry-over contamination using Uracil-DNA Glycosylase (UDG)
• Application of IPC system to verify the validity of PCR
| No. | Kit Contents | Unit |
|---|---|---|
| 1 | 2X pPCR mix | 280 μl x vials |
| 2 | Detection solution | 140 μl x 2 vials |
| 3 | Positive control | 25 μl x 3 vials |
| 4 | DNase/RNase free water | 1,000 μl x 1 vial |
| 5 | Manual | 1 ea |
• 12 months from manufacturing date
• Stable Within 6 months after opening, within expiry date of the kit

1. Sensitivity
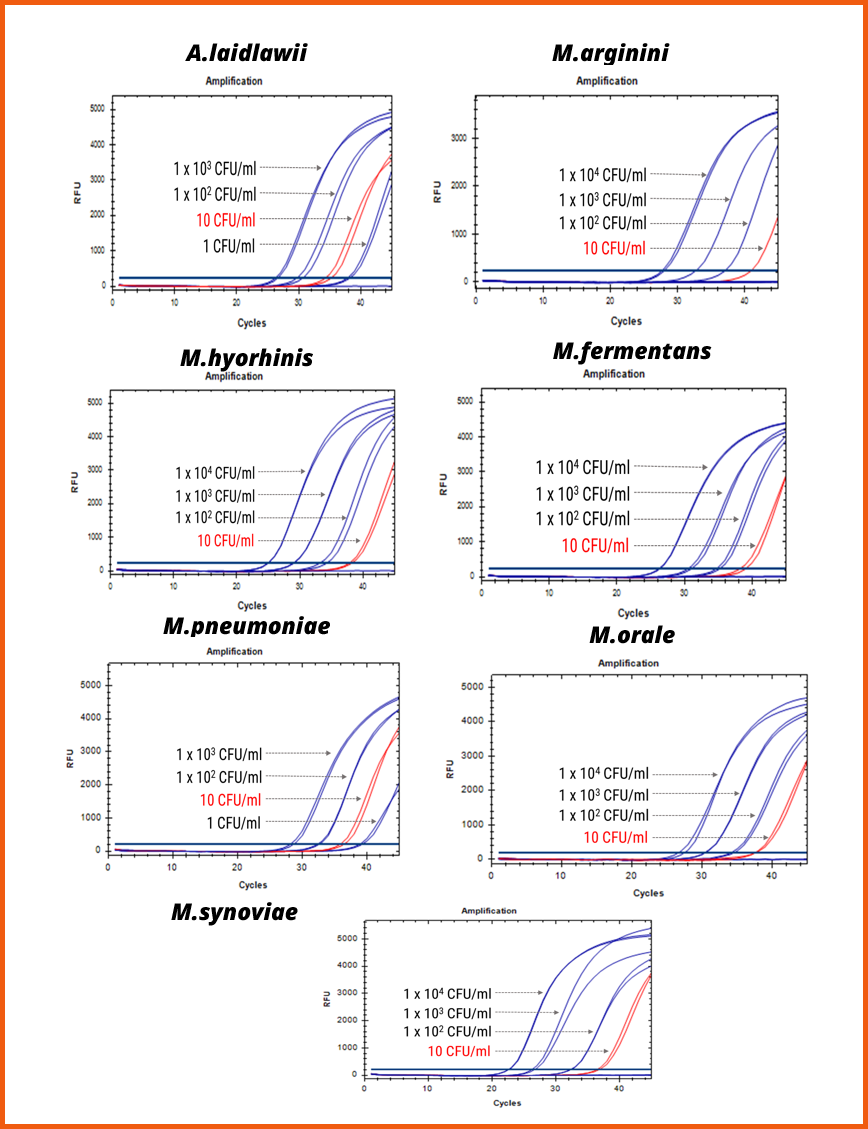
[Evaluation of the e-Myco™ VALiD-Q Real-time PCR Kit (ver. 2.0) of the detection limits for 7 types of Mycoplasma genomic DNA]
• The e-Myco™ VALiD-Q Real-time PCR Kit (ver. 2.0) evaluated the detection limits of the product by extracting nucleic acids from culture media obtained through direct culture of 7 Mycoplasma species.
• The detection limit was confirmed to be below 10 CFU/ml for all Mycoplasma genomic DNA.
• The experiment involved diluting genomic DNA at a concentration of 1x10,000 CFU/ml to a final concentration of 1 CFU/ml, and the detection results were confirmed for five stages of concentration gradient.
2. Compatibility of FaSTARamp96 Real-time PCR system
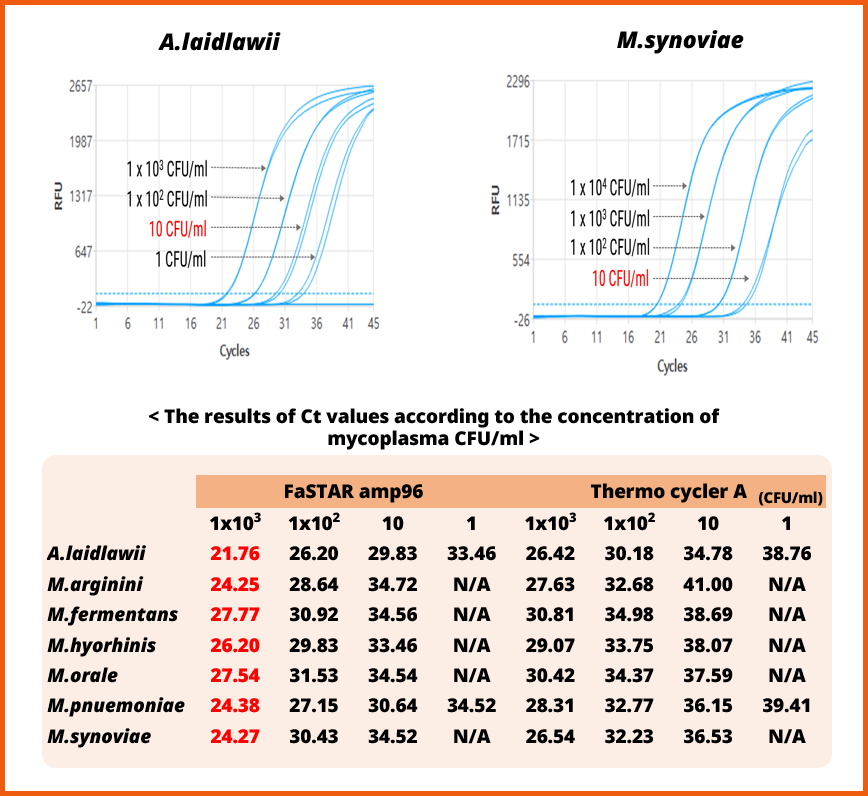
[Evaluation of compatibility of the e-Myco™ VALiD-Q Real-time PCR Kit (ver. 2.0) with the FaSTARamp96 Real-time PCR System]
• The e-Myco™ VALiD-Q Real-time PCR Kit (ver. 2.0) was evaluated for its detection limits using iNtRON's real-time PCR equipment, the FaSTARamp96 Real-time PCR System.
• The genomic DNA of 7 Mycoplasma species was diluted from 1x1000 CFU/ml to 1 CFU/ml, and the concentrations for four dilution steps were confirmed.
• It was found that the detection limits for the 7 Mycoplasma species were consistent with those of other equipment, while also demonstrating the capability to detect Ct values more than 3~4 cycles faster from the same concentration of template.
3. Specificity
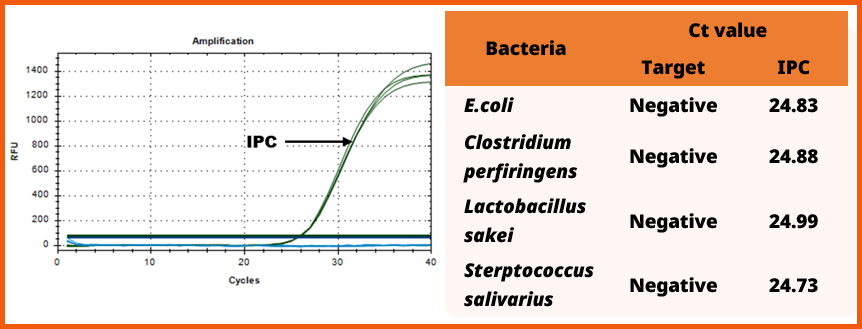
• The evaluation of specificity for the genomic DNA of Clostridium, Lactobacillus and Streptococcus
• To evaluate the specificity of the e-Myco™ VALiD-Q Real-time PCR Kit (ver. 2.0), nucleic acids were extracted from related mycoplasma strains : Clostridium(C. perfringens), Lactobacillus(L. sakei), Streptococcus(S. salivarius), using genomic DNA at a concentration of 10 ng/µL.
• As a result, negative results were confirmed, and the amplification of the IPC was also observed within the normal range of 22 to 27.
4. Performance
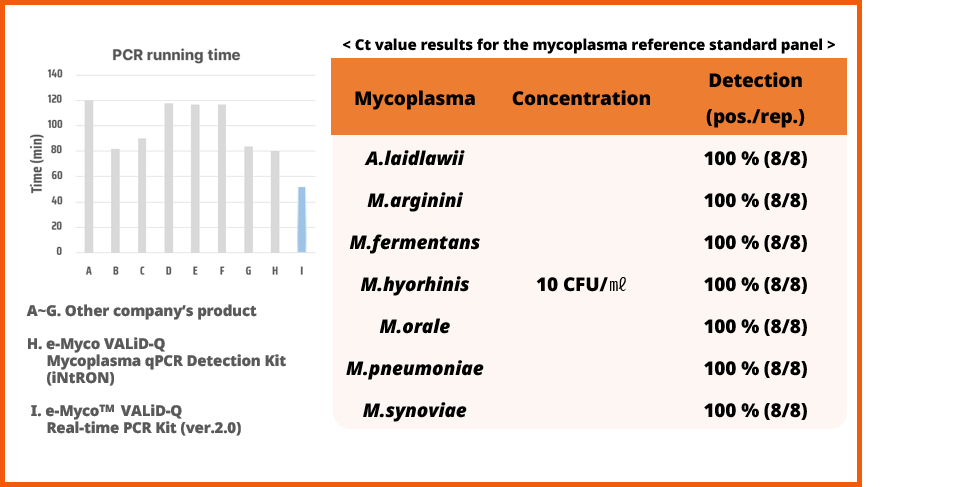
• Evaluation of Mycoplasma Detection Performance at 10 CFU/ml under Rapid Testing Conditions.
• The e-Myco™ VALiD-Q Real-time PCR Kit (Ver. 2.0), which enables Mycoplasma testing within one hour, is over 60 minutes faster than competitors and has been confirmed to achieve 100% detection using a certified reference standard panel of mycoplasma at 10 CFU/ml.
| Product | Cat.No | Capacity | inquire | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FaSTARprep96 Automated Nucleic Acid Extraction System Best |
|
|||||||||
| FaSTAR-XT PGS DNA/RNA Kit (I) |
|
|||||||||
| FaSTAR-XT PGS DNA/RNA Kit (W) |
|
|||||||||
| e-Myco™ VALiD Mycoplasma PCR Detection Kit Best |
|
|||||||||
| e-Myco™ VALiD-Q Mycoplasma qPCR Detection Kit Best |
|
|||||||||
| G-spin™ Total DNA Extraction Mini Kit Best |
|
|||||||||